Guidance for submission and distribution of educational material
Published:
|
Updated:
Changes
- : Added section "GVP Module XVI Rev. 3 update"
- : Updated rules concerning version number and date
- : Text stating that MAH for a generic product must ensure that healthcare professional are aware of educational material by sending emails or letters. It must be specified that materials can be ordered from the MAH
- : Online course has been removed
- : The entire text has been reviewed and several revisions have been made to enhance clarity and readabilityimprove language
- : The section on the submission of educational materials for over-the-counter medicines with guidance has been removed
- : The diagram outlining when the MAH should submit educational materials has been updated to align with new graphic profile.
- : The checklist has been updated according to new GVP Module and new graphic profile.
- : Added point "the regulatory procedure which has led to the requirement of educational material" under "The following must be submitted before use and distribtion"
- : Updated rules concerning use of company logo
- : The text regarding exceptions for the black triangle on patient cards has been removed
- : Added section "Title, product name and contact information" and "Patient card"
- : Updated link to GVP Module XVI- Risk minimisation measures due to new revised version (Rev 3). Also deleted link to GVP Module XVI- educational material as this is now incorporated in GVP Module XVI-Risk minimisation measures (Rev 3)
- : Amended recommendation for the distribution of educational material for generic products
Marketing authorisation holders may be required to make material for the education of healthcare professionals, patients and relatives for medicines which have a special risk of adverse drug reactions. The purpose of the material is to reduce these risks.
Page contents
Educational material is a type of additional risk minimisation measure (aRMM), and the requirements for these aRMMs is given during the processing of the application for the marketing authorization. For medicinal products in central procedure, the requirements are given in Annex II, for other procedures they are described in the risk management plan (RMP).
This figure gives an overview of when the MAH should submit material to the Norwegian Medical Products Agency:
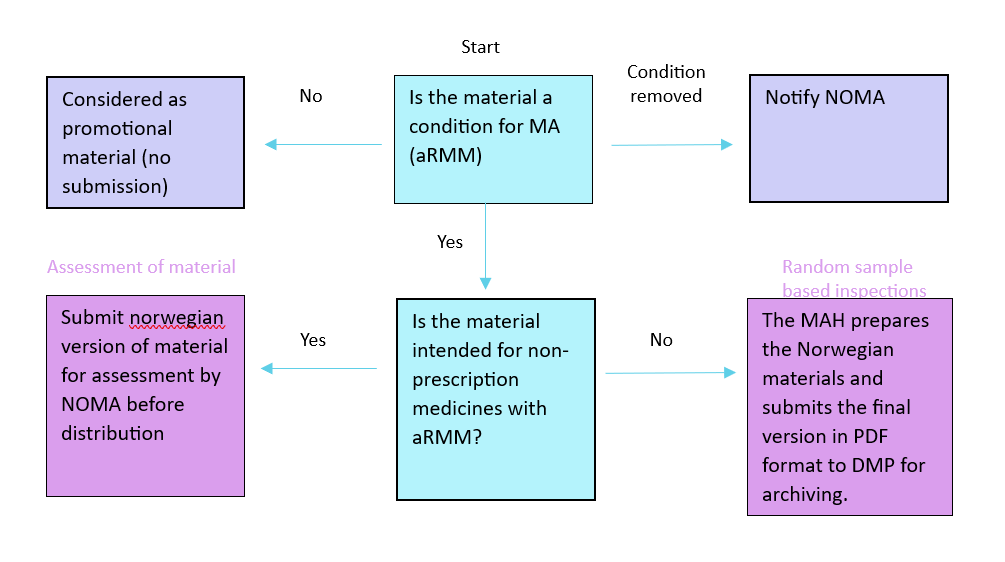
Submission of educational material
Educational material with random sample-based inspections
In November 2020, NOMA introduced sample-based inspection of educational materials. Under this arrangement, the MAH must submit final Norwegian versions of educational material before distribution. NOMA does not assess the material before use. The material is archived internally and NOMA conducts sample-based inspections. The inspections ensures that the educational material comply with EMA recommendations and national guidelines. When educational material are selected for sample-based inspection, the MAH will receive feedback after assessment.
A checklist and glossary are available to assist in the preparation of educational material.
Submit the following before the use and distribution of educational material:
- Final Norwegian PDF versions of material with final layout (and possibly cover letter)
- Latest version of the risk management plan (RMP) part V (for CP-products can annex IID of SmPC also be used)
- the regulatory procedure which has led to the requirement of educational material.
- Target groups (MAH determines this themselves)
- Distribution plan including schedule for distribution
In addition, submit the following when updating material:
- justification for updating the material
Send the material to opplaeringsmateriell@dmp.no or educationalmaterial@noma.no
Medicinal products that is already marketed, and receives a new aRMM-requirement
For products that are already marketed, and receives a new aRMM-requirement, ie. an update to the Annex IID, the submission deadline for educational materials are 30 days after the requirement is established.
Guidance
Guidance for the development of educational material:
EMA's overall description of various risk minimization measures and educational material:
Guideline on good pharmacovigilance practices (GVP) Module XVI - Risk minimization measures (Rev. 3)
Checklist for the development of ecucational material
Follow national guidelines available on this website
GVP Module XVI Rev.3 update
In august 2024, GVP Module XVI version 2 was replaced by version 3. Following this NOMA has updated the webpage "Guidance for submission and distribution of educational material". The updates includes changes based on the new version of GVP XVI (Rev. 3). In addition NOMA has reviewed and updated the national requirements and recommendations.
The updated guidance applies to new educational materials developed after the publication of GVP Module XVI version3. The MAH is not required to update existing materials to comply with the new guidance. However, when the MAH needs to update existing educational material for other reasons, the materials should also be updated to align with the new guidance.
Text and language
The educational material must be in Norwegian. The language must be clear and concice, avoiding heavy and complex sentences. If the quality of the language is poor or incomprehensible, the MAH will be asked to improve the language after sample-based inspection.
All points described in RMP Part V/Annex IID must be addressed.
All unnecessary text in educational material should be avoided, as the purpose of educational material is to describe specific risks associated with the medicinal product.
Examples of unecessary text include mechanisms of action, disease pathology, and similar content.
Use of color, image and logo
Educational material must not contain colors that are commercially related, i.e. colors that are related to the marketing of the product. However, it is allowed to use the MAH's colors in the material.
As a general rule, the MAH should avoid using company logo and product logo in the material. If the MAH considers it appropriate to include a logo, a justification must be provided. The logo, if included, should appear only once in the materials and must not be larger than the font size of the title.
Images or illustration should only be included if they enhance understanding of the content, such as illustrating specific procedures, injection sites, administration techniques, or similar.
The use of color must not reduce readability due to poor contrast.
Signature from prescriber/patient
Educational material must not include signature lines or require signature from the prescriber and/or patient unless this has been mandated by EMA/the Commission. Signature fields may give the wrong impression of additional obligations for the patient or healthcare professional who signs.
This does not prevent the inclusion of contact information of the treating physician/clinic or similar details in patient material, provided the intent is to inform other healthcare personnel in the event of a crisis situation for the patient.
Version number
Educational material must include a version number and submission date in the format <month><year>, at a minimum on the first and/or last page, but preferably on all pages.
Cover letter
It is recommended that the MAH includes a cover letter with the distribution of the material, explaining the purpose of the educational material or any changes. The MAH should mark the cover letter with DMP's standard logo for safety information.
Title, product name and contact information
The title should contain type of material, such as whether it is a guide for healthcare personnel or patients. It should also include the product name with the active ingredient name in parentheses. The product name should be used as few times as possible in the rest of the material.
Contact information to MAH, such as an email adress or phone number, must be included in the educational material.
Safety information logo
The Norwegian Medical Products Agency's safety information logo is a national requirement. The logo must be included on educational material, cover letters, and envelopes so that healthcare personnel and patients can distinguish between educational and promotional material. For patient cards, the MAH must assess whether there is enough space.
Please note that the safety infomation logo for educational material is different from the safety information logo used for Direct Healthcare Professional Communication (DHPC).
Standard text
Reporting of adverse drug reactions
Educational material must include text encouraging the reporting of adverse reactions. For patient cards, the MAH must assess whether there is enough space.
The following standard texts should be used:
Text for material to healthcare professionals
Helsepersonell bes melde nye, uventede og alvorlige mistenkte bivirkninger på elektronisk meldeskjema: www.dmp.no/meldeskjema
Text for material to patients
Bivirkninger kan meldes på elektronisk skjema til DMP: www.dmp.no/pasientmelding
For medicinal products with a black triangle, specific standard texts are provided in the Black triangle section.
Standard statement of where educational material can be found online
It should be stated in the educational material and cover letter, where the educational material, package leaflets and the SmPC can be found online. The following standard phrase can be used:
Se oppdatert pakningsvedlegg, preparatomtale (SmPC) og opplæringsmateriell på www.felleskatalogen.no.
Black triangle
Educational materials for medicinal products on the common European watch list shall contain a black triangle in front of product name ( ▼Product name) at the beginning of the material. The triangle must have the same height as the letters in the product name it appears in front of. On the same page, an explanatory text about the black triangle should be include (See GVP Module X C3.5 for details).
Explanatory text about the black triangle for:
Healthcare professionals
Dette legemiddelet er underlagt særlig overvåking for å oppdage ny sikkerhetsinformasjon så raskt som mulig. Helsepersonell oppfordres til å melde enhver mistenkt bivirkning på elektronisk meldeskjema:
www.dmp.no/meldeskjema
Patients
Dette legemiddelet er underlagt særlig overvåking for å oppdage ny sikkerhetsinformasjon så raskt som mulig. Du kan bidra ved å melde enhver mistenkt bivirkning:
www.dmp.no/pasientmelding
Distribution
The MAH must submit a proposal to NOMA on how the material will be distributed/published. A timeline for publishing and/or distribution must also be proposed. As a general rule, new educational material should be distributed to selected target groups, in addition to being published on Felleskatalogen. This also applies to significant changes in existing educational material. In the event of minor changes, it may be sufficient to publish the material only on Felleskatalogen.
Educational material for generic medicines are generally not distributed. The MAH of the origin product has often already distributed the educational material. However, the MAH for generic medicines must ensure that prescribers are aware that educational material for their product exists by sending emails or letters. It must be stated that the physical copies of the material can be ordered from the MAH.
Pharmacy
Educational materials for HCPs in pharmacies are sent by e-mail. An overview of email addresses for pharmacies is available from the Norwegian Pharmaceutical Product Compendium (Felleskatalogen). To receive the lists, send an e-mail to redaksjonen@felleskatalogen.no with the subject field "E-post apotek til utsendelse av opplæringsmateriell".
The email to the pharmacies should contain the text "Sikkerhetsinformasjon i samarbeid med DMP” in the subject field. Attach the educational material to the email in PDF format.
General practitioners
Educational material should not be sent to GPs by post mail. The Norwegian Medical Products Agency instead disseminates the information through notifications in the electronic patient journal (EPJ).
Updated material is only notified through this system if the update is required by the authorities. It is therefore important that it is clearly stated in the submission to the Norwegian Medical Products Agency whether the update is required by the authorities. In addition, clearly state that GPs are the recipient group for the material.
Other physicians/other healthcare professionals
Educational materials for other groups of physicians/health personnel must be sent by post mail.
Publication at Felleskatalogen
We strongly encourage publishing all educational material in the Norwegian Pharmaceutical Product Compendium (Felleskatalogen). The reason for this is that almost all health personnel use Felleskatalogen, and there is a greater probability that educational material will be used if it is easily accessible. It will therefore increase patient safety if all educational materials are available to healthcare professionals and patients through Felleskatalogen.
There are three categories of educational materials on Felleskatalogen:
- Educational material and instructions for use
- Pregnancy prevention program
- Educational material and pregnancy prevention program
Felleskatalogen groups the material into the correct category when the material is submitted to them.
- Educational material (RMP material) is published on www.felleskatalogen.no
- Use FK SHARE for uploading, https://share.felleskatalogen.no/
- Are you a new FK SHARE user? Send e-mail to redaksjonen@felleskatalogen.no to be registered
NB! RMP material should always be marked with ‘RMP’ in the file name. Since RMP materials are a requirement by NOMA, the editorial team at Felleskatalogen will not assess this material further.
Educational material for generic products
Where the conditions for generic products are identical to the original product, the educational material should be harmonised with the existing material for the original product.
For the distribution of educational materials for generic product, see the distribution section above.
Joint material and distribution
Marketing authorization holders for different products with the same substance and identical conditions concerning educational materials are encouraged to collaborate on the preparation of the educational material. The MAH should submit only one version for the educational material to the Norwegian Medical Products Agency. We also recommend that the marketing authorization holders collaborate on the dissemination of the educational material to make sure that the recipients on the distribution list receive the same common educational material.
Online courses
The online courses were outdated due to the new version of GVP Module XVC Risk Minimisation Measures (Rev. 3) and therefore removed. It is under consideration whether new videos should be made and whether the course should be continued.